
There are instances when the histology laboratory will receive a hair specimen from a patient for diagnosis. This is not to be confused with residual hair left on a skin specimen. Sometimes, hair itself is obtained by pulling it from the scalp, in order to determine any pathology that might be present.
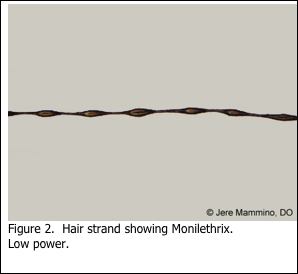
Hair specimens for genetic disorders are usually submitted unfixed. In these cases, the pathologist will require that the hairs simply be submitted on a microscope slide with a coverglass, held in place with a drop or two of mounting medium to keep the hair in place. The hair is then viewed by the pathologist, unstained, under light microscopy. An example of such a preparation is shown in Figures 1 and 2. Monilethrix is a disease that may also be referred to as “beaded hair”. Patients present with short, fragile broken hair. This condition is caused by a genetic mutation for which there is no treatment.
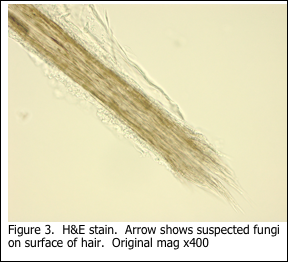
More commonly, hair specimens may be submitted to the histology laboratory for diagnosis of trichomycosis, which is a general term for fungal infection of the hair.
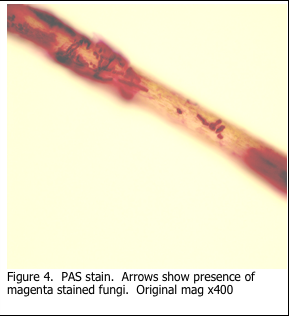
In the previous blog, 2015 #4, the use of the periodic acid Schiff’s (PAS) stain to stain mucins was discussed. Additionally, it was noted that the Schiff’s stain is not specific for glycogen. Schiff’s reagent will combine with any aldehyde groups to form pink reaction product. Thus the PAS stain can be used to stain fungi, in addition to mucin and glycogen. The sugars present in the fungal cell wall are oxidized and react with the Schiff’s reagent to form the pink reaction product. Digestion with diastase does not remove the staining.
There are some technical challenges presented when a hair specimen reaches the histology laboratory and requires a PAS stain for fungi. The hair strands must be handled gently with forceps, as the surgical grossing is performed and the specimen described. The specimen should not be processed into a paraffin block for routine H&E staining. Instead, the hairs should be transferred to a 20 ml glass scintillation vial (or similar container) in order for the PAS stain to be performed manually. The staining solutions are transferred by using a disposable 3 ml plastic bulb pipette. In this way, the hairs are retained in the container. Solutions should be discarded into a beaker, in order to prevent any of the hair strands from being lost. The detailed procedure is provided below.
PERIODIC ACID SCHIFF’S ON HAIR SHAFTS
PRINCIPLE: The periodic acid-Schiff reaction (PAS) detects tissue carbohydrates, particularly for glycogen when used with a diastase digestion. Use of diastase digestion prior to the reaction will remove glycogen. This is important when attempting to localize fungi, whose cell walls are carbohydrate. Thus the PAS reaction can be used to localize both glycogen and fungi. Fungi are detected both with, and without, diastase digestion.
SPECIMEN: Formalin preferred. Other fixatives may be used. 5 micron paraffin sections.
QUALITY CONTROL: Include a known positive control with fungal tissue.
PROCEDURE:
- Confirm the number of hair shafts present in the specimen bottle.
- Pour off the formalin fixative into a beaker.
- Rinse with distilled water for 5 minutes.
- Pour off into the waste beaker.
- Add enough 0.5% periodic acid to cover the hair shafts.
- Incubate for 10 minutes. Pour off into the waste beaker.
- Rinse with distilled water for 5 minutes.
- Add enough Schiff’s reagent to cover the hair shafts.
- Incubate for 20 minutes. Pour off into the waste beaker.
- Rinse with distilled water for 2 minutes. Pour off into the waste beaker.
- Rinse with tap water 3 times 3 minutes. Pour off into the waste beaker.
- Leave specimen cap off and allow to air dry for 30 minutes.
- Label a microscope slide with the accession number, patient name, PAS, and date.
- Mount the hair shafts on the slide with Permount, and a cover glass.
RESULTS:
Fungi will appear as bright pink strands on the hair shaft, if present.
REAGENTS:
1. 0.5% Periodic Acid (Oxidizer) – make fresh before use
2. Schiff’s Reagent
REFERENCES:
- Theory and Practice of Histological Techniques. JD Bancroft, A Stevens ed. Churchill Livingstone, NY. Fourth edition. 1996
- Theory and Practice of Histotechnology. DC Sheehan, BB Hrapchak. CV Mosby Company, St. Louis. First edition. 1980.
- Human Hair Atlas – Microscopic. www.swgmat.org
- Dr. Terence J. Harrist – personal communication
- Dr. Geoffrey Gottlieb – personal communication


