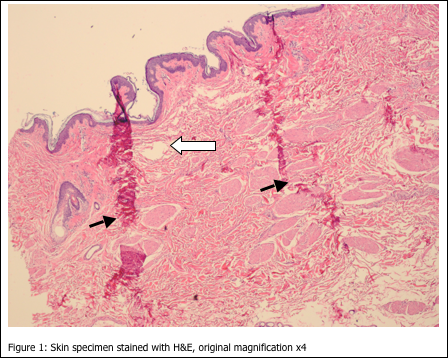
The artefacts shown in Figure 1 are most likely due to:
- incomplete fixation
- improper embedding
- incomplete dehydration
- incomplete infiltration with paraffin
The most noticeable artefacts in Figure 1 are the wrinkles in the tissue sections (closed arrows). In addition, there are multiple holes and tears throughout the section (open arrow). Incomplete fixation would not cause such artefacts, as the cellular histology is acceptable (i.e. nuclear and cytoplasmic staining is within quality control limits). Improper embedding would also not be the cause of these artefacts. When skin specimens are mis-embedded, usually tearing and wrinkles would be localized within the epidermis, and within the dermal-epidermal junction.
The most likely cause of the artefacts seen in Figure 1 is a combination of c and d, incomplete dehydration, resulting in incomplete paraffin infiltration. When tissue is not completely dehydrated, excess water is left in the tissue. If xylene is used as the clearing agent, it can dissolve up to 2% water – but no more. Also, if xylene substitutes are used, they are completely intolerant of any residual water. If water is present in the tissue after dehydration and clearing, paraffin cannot infiltrate the tissue completely. Without complete paraffin infiltration, the tissue can tear and fold during microtomy. If this affects all tissues in the run, the tissues may have to be reprocessed by melting down the blocks and placing the tissues back into the cassettes, with new cassette tops. The cassettes would then be ran back through changes of xylene and 100% alcohol. Finally, they would be ran back through xylene and into paraffin. This procedure should remedy the problem.
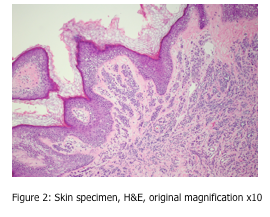
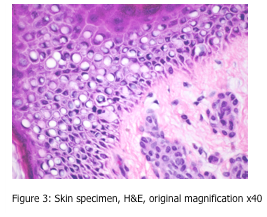
Can you spot the artefact shown in Figures 2 and 3?
Under both low and high power magnification, one can observe vacuolization of many cells, sometimes referred to as the “swiss cheese effect”. These large, empty circular spaces are located within cells, and show some nuclei to be pushed to one side of the cell. This is an artefact sometimes seen in northern latitudes, as it is caused by slow freezing of the tissue specimen. During this slow freezing process, ice crystals can form within the cell. As we all know, when water changes to ice, it expands and takes up more space. These ice crystals are the cause of the resulting empty spaces within the cell.
This artefact is not observed in the “rapid freezing” of fresh tissue in a cryostat. The tissue should be frozen so quickly, that ice crystals do not have an opportunity to form. If you are observing this artefact in your cryostat sections, the tissue is not being frozen rapidly enough.
Reference laboratories operating in northern climates can help prevent the formation of artefacts by providing formalin fixative that contains up to 10% ethanol. Addition of the ethanol helps to decrease the freezing point of the fixative/ tissue. It is not recommended to add ethanol in a percentage greater than 10%, as it may cause the tissue to exhibit “foamy nuclei” – bubbles within the nuclear material. This artefact results from the tissue undergoing fixation from both formaldehyde and ethanol at the same time. These chemicals have different fixation chemistry, which occur at different times, and can result in the “foamy nuclei” artefact.
REFERENCES:
- Theory and Practice of Histological Techniques. JD Bancroft, A Stevens ed. Churchill Livingstone, NY. Fourth edition. 1996
- Theory and Practice of Histotechnology. DC Sheehan, BB Hrapchak. CV Mosby Company, St. Louis. First edition. 1980.



