In the previous segments of the IHC Educational Series, immunofluorescence and immunoperoxidase methods were discussed. In both of these methods, antibodies are used to bind to and localize protein antigens in tissue sections. Additionally, the differences between the use of polyclonal and monoclonal antibodies had been described.
Another immunohistochemical method is “in situ hybridization”. The term “in situ” means “in the original place”, while “hybridization” refers to a hybrid composed of one strand of DNA with a complementary strand of nucleotides – which is referred to as a probe. Thus “in situ hybridization” describes a technique whereby a single strand of nucleotides is used as a probe to recognize and bind to its complementary strand of DNA, located “in place” within the nuclei of cells present in a tissue section.
Double stranded DNA is built from simple building blocks of four amino acids: adenine, thymine, cytosine and guanine. Adenine always binds with thymine and cytosine always binds with guanine. DNA can “unwind” in the nucleus to provide a template to construct complementary RNA (“transcription”). The RNA then exits the nucleus to bring the “message’” into the cytoplasm, binding with ribosomes to manufacture proteins. Figure 1 illustrates this principle.
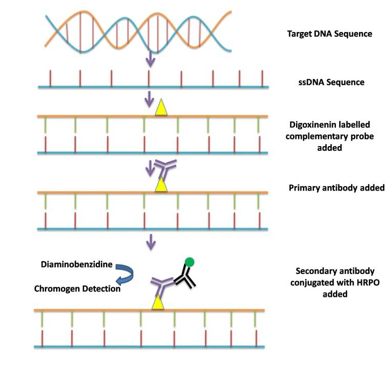
When double stranded DNA within a tissue section is heated to approximately 95 C in the presence of an appropriate buffer, the strands separate. Once separated, a nucleotide probe (attached to a label) can be placed on the slide and incubated. The probe will bind to its complementary strand of DNA. Upon cooling, the DNA strand will close back up.
Labels, or “reporter molecules” can be radioactive molecules. However, in histology, usually biotin, digoxigenin or fluorescent labels are used. If fluorescent molecules are used, the method is referred to as “FISH”: fluorescent in situ hybridization. The staining results are viewed in a darkfield fluorescence microscope. If biotin or digoxigenin is used in conjunction with a chromogen label, the method is referred to as “CISH”: chromogenic in situ hybridization. These slides can be viewed using a standard routine light microscope. A schematic diagram of the procedure is shown in Figure 2.
What are these probes recognizing? Researchers can construct very specific probes that will recognize and bind to very specific DNA sequences within the nuclei of cells. In this way, specific gene sequences can be recognized within a histologic section of tissue. The results can help identify cells which are abnormal and/or may eventually give rise to cancer cells.
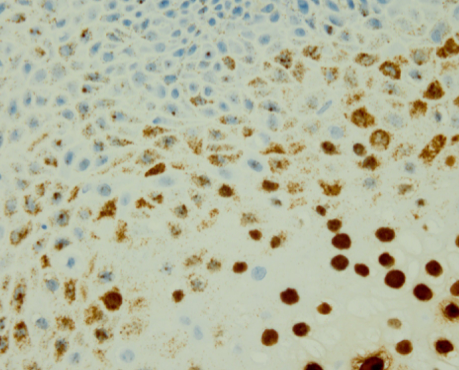
One such example is the use of probes in the diagnosis of human papilloma virus (HPV) in head and neck cancers. The literature offers information that the presence of E6/E7 viral oncogenes in biopsies is evidence of the presence of high-risk HPV. HPV biomarker detection reagents can be used to demonstrate these oncogenes in formalin fixed paraffin embedded tissue sections. Additionally, these probes can be directed against the mRNA contained in the section. Positive staining indicates the presence of transcriptionally active mRNA, providing increased sensitivity of the method. This activity can be demonstrated even when the actual viral load is very low. Figure 3 shows positive in situ hybridization staining for HPV low risk strains.
Clearly, one aspect of the future of histology is down the path of molecular biology. Information can now be obtained regarding gene location in the nucleus, and mRNA found in the cytoplasm. This information is valuable in helping clinicians to formulate prognoses and treatments for their patients.
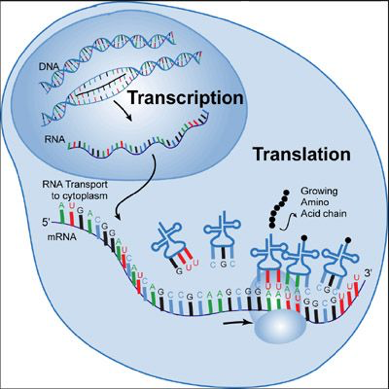
IHC5 Figure 1 
IHC5 Figure 2 
HPV low risk positive staining shown by dark brown staining against blue nuclear counterstain. Original magnification x600.