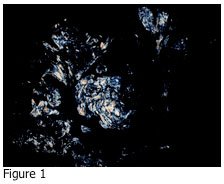
Please review the photograph shown in Figure 1. The image shown in Figure 1 is:
- specific fluorescein staining
- amyloid deposits
- specific rhodamine staining
- urate crystals
If you answered A, you are incorrect. Fluorescein is a labelling molecule attached to antibodies to stain specific antigens. Fluorescein staining appears as very bright, green staining where antibodies have attached to their specific antigens. The black background is correct for a fluorescence micrograph due to viewing the slides in a fluorescence darkfield microscope. However, the specific staining shown is white, not green.
If you answered B, you are incorrect. Amyloid deposits stained with Congo red would appear as apple green birefringence against a dark background of polarized light.
If you answered C, you are incorrect. Rhodamine is also used as a labelling molecule in immunofluorescence staining. However, when excited by ultraviolet light, the molecule fluoresces red, against a black background.
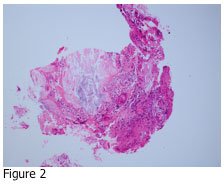
If you answered D, you are correct. Urate crystals may precipitate out in the joint fluid and tissues of patients who have elevated levels of uric acid in their blood. Figure 2 shows the adjacent tissue section of Figure 1 stained with hematoxylin and eosin (H&E). The tissue is a skin biopsy taken from a patient with suspected gout disease, which results from the elevated uric acid in the blood. If the concentration of uric acid gets high enough, it may precipitate out in tissues as urate crystals. As you can see from Figure 1, these crystals are very sharp, and can cause pain in the affected joints and other areas.
The only way the crystals can be preserved for histological identification by polarizing microscopy is for the tissue to be fixed in Carnoy’s fixative (i.e. chloroform and absolute ethanol) or one hundred percent ethanol. Fixation in 10% formalin, or any other fixative that contains water, may cause the crystals to dissolve out of the tissue, causing a false negative result. Therefore, it is imperative that the clinician performing the biopsy be provided with this information, and the proper fixative.
Once the specimen is fixed, it must be specially processed to avoid any further exposure to water. Typically, the specimen is transferred from the Carnoy’s fixative (or one hundred percent ethanol, if that is used to fix the tissue), to fresh one hundred percent ethanol to begin processing. Subsequent transfers to xylene and molted paraffin complete the processing.
Embedding is performed according to the type of specimen received. A routine H&E slide is prepared for review by the pathologist, as well as an unstained section. The unstained section slide is simply soaked in xylene to remove the paraffin, and then cover slipped. This slide is observed with a light microscope under polarized light. In this instance, there must be two polarizing filters present: one under the slide, and one above the slide. The filter below the slide (typically situated right over the light source) is turned until all light is obscured. Any urate crystals that are present will appear as bright, white crystals.
In a similar manner, your histology laboratory may receive a syringe containing “joint fluid” for crystal analysis. The principles detailed above apply to this specimen type as well. CAUTION! The syringe will contain unfixed patient fluid, possibly containing blood borne pathogens. These specimens should be handled with Universal Precautions, using all personal protective equipment (i.e. PPE) in a properly ventilated work space.
The fluid from the syringe is simply pushed out onto a properly labelled microscope slide (i.e. with two patient identifiers), and left lying flat in a ventilated hood to air dry. Once dry, a drop of Permount is added, and the slide cover slipped. The slide is then observed as described above. Urate crystals present will be easily identified.
Similarly, specimens for immunofluorescence require use of a specialized fixative. They must not be fixed in formalin. These specimens are usually designated for the procedures of direct immunofluorescence. The specimens must be fixed in Michel’s transport medium, or any similar “immuno transport” solution. These fixatives are composed of solutions of ammonium sulfate, which help to stabilize and fix proteins in place. If formalin is used, the antigenicity of these proteins becomes destroyed due to the cross linking properties of the formalin, rendering the specimens unusable for the fluorescence procedures.
Specimens that are submitted for detection of the presence of amyloid may be fixed in 10% formalin and processed routinely. However, during microtomy, they must be cut at a thickness of 8 – 10 microns. Also, any unstained slides, or positive control slides, should be stored in the refrigerator.
In summary, it is imperative to identify these unique specimen types upon receipt in your laboratory before they are routed to routine processing. Vigilance at this step will save you the possibility of rendering a specimen unfit for further processing.


