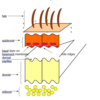
CelLock TM: An innovative standardized cell-block preparation procedure
Histology Educational Series In the previous blog we saw that every histology laboratory has a method for preparing paraffin cell-block specimens, and we reviewed those methods. This review showed that …