When staining sections for the presence of carbohydrates, the two main classes under investigation are glycogen and mucins. Mucins include substances referred to as mucopolysaccharides, mucosubstances, glycoproteins and glycoconjugates.
Mucins provide an environment that is conducive to molecular diffusion of chemicals, especially those in ionic form. Mucin also increases the binding between cells, and may help to “shed’ bacteria and viruses. The demonstration of the presence and absence of mucins within tissue sections is an important tool to assist pathologists in rendering a diagnosis. The following types of mucins can be distinguished by certain special stains.
Acidic mucins contain sulphur in varying amounts. Other acidic mucins can have carboxyl groups attached to them, which make them acidic.
Neutral mucins, as the name implies, contain no acidic reactive groups within. These mucins are epithelial in origin and are commonly found in gastric lining cells.
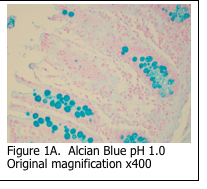
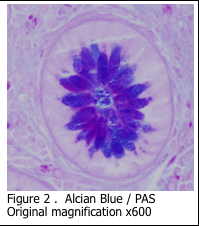
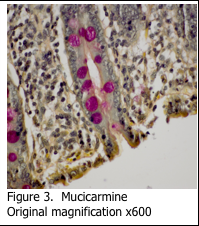
Alcian blue is the most common stain used for demonstrating the presence of acidic mucins, since the blue dye molecules are cationic (positively charged) and bind to the anionic sulphur and carboxyl groups within the mucin. Neutral mucins are not usually stained by alcian blue. The pH of the alcian blue solution can be adjusted to stain different classes of acidic mucins (Figure 1A and 1B). Alcian blue can also be combined with a PAS stain to show both acidic and neutral mucins (Figure 2). Mucicarmine is sometimes used to demonstrate acidic mucins (Figure 3). Mucicarmine also will stain encapsulated fungi such as Cyrptococcus neoformans.
  |  |  |
 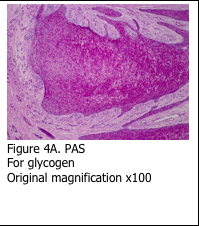 |  |
Glycogen is the cellular mode of storing sugar, which is in the form of a six-sided molecule. Glycogen can be demonstrated histologically using the periodic acid – Schiff’s stain (PAS). The glycogen molecules are oxidized by the periodic acid, which breaks the ring and exposes aldehyde groups. Subsequent exposure to Schiff’s reagent causes the aldehyde groups to react with it and form a pink reaction product (Figure 4A).
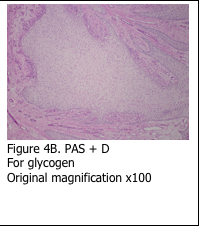
Presence of glycogen is confirmed by staining a serial section of the tissue with periodic acid – Schiff’s plus diastase (PAS+D). The section is incubated with diastase solution prior to performing the PAS stain – in order to digest all of the glycogen. The end result is the absence of pink stain (Figure 4B).
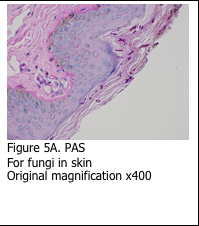
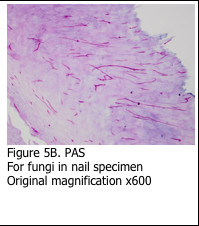
It is important to remember that the Schiff’s stain is not specific for glycogen. Schiff’s reagent will combine with any aldehyde groups to form pink reaction product. This is evident in using the PAS stain to identify the basement membrane in skin specimens (Figure 5A). Additionally, the PAS stain can be used to stain fungi (Figure 5B). The sugars present in the basement membrane and the fungal cell wall are oxidized and react with the Schiff’s reagent to form the pink reaction product. Digestion with diastase does not remove the staining.
References
- Theory and Practice of Histological Techniques. Chapter 10. JD Bancroft, A Stevens ed. Churchill Livingstone, NY. Fourth edition. 1996
- Theory and Practice of Histotechnology. Chapter 9. DC Sheehan, BB Hrapchak. CV Mosby Company, St. Louis. First edition. 1980.


