Why even bother to learn about troubleshooting? Wouldn’t it be more interesting to listen to one of the presentations on molecular technology? Possibly. But what would you do if your pathologist brought you a slide from the first batch of today’s H&E’s that looked sub-optimal to review alongside an optimal slide stained the previous day?
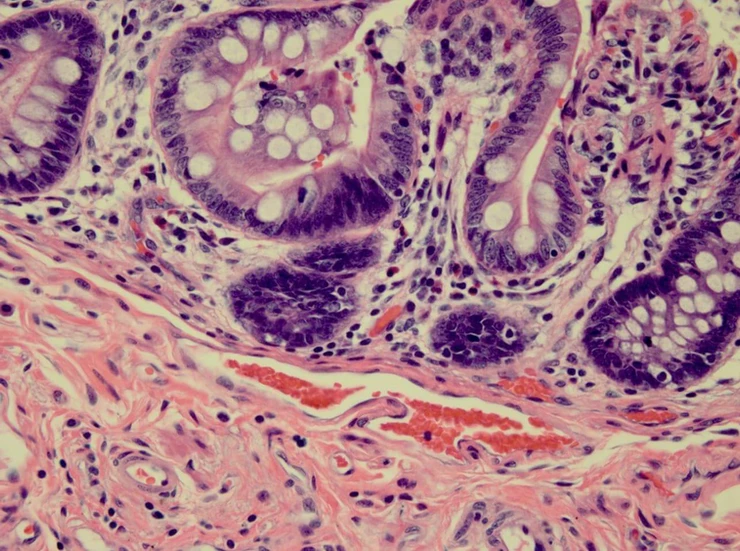
Pathologist: “Can you see that this slide from yesterday is exceptionally good? The nuclei are stained dark blue, and you can see nuclear detail. The eosin is staining in three distinct shades of pink. This is a really excellent slide.”
You: “Yes, I can see that. Thank you.”
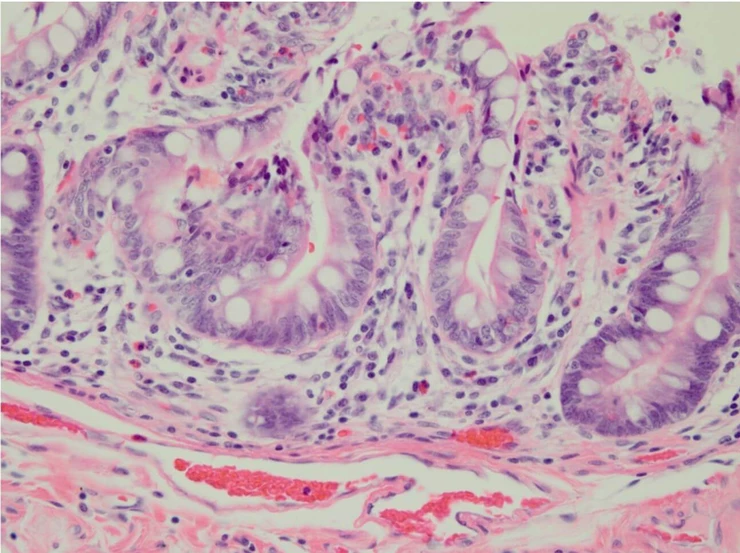
Pathologist: “Now, can you see that this slide from today is very pale? The nuclei are stained a faint blue and you can’t see nuclear detail. The eosin appears washed out. This is an awfully bad slide, and I cannot make a diagnosis from it.”
You: “Yes, I can see the problem.”
Pathologist: “Great. Since you can see the problem, please fix it. You can start by fixing all the slides that are sitting on my desk from today. I will need them ASAP. Then please make sure that this doesn’t happen again.”
You: “OK. I will get right on it.”
Well, you can “Get right on it” – but only if you know and have a method for troubleshooting. This is a complex issue- it could have originated during tissue processing, microtomy and/or staining. You can’t “fix it” if you can’t isolate the exact cause and come up with a solution.
Your first step in troubleshooting should be to methodically assess the situation for the exact cause of the suboptimal stain. Questions to ask might include:
1. What happened between yesterday and today?
- Where there any personnel changes/ task changes for personnel?
2. Something is wrong with the stain – so it must be the stainer – (right?)
3. Or…. did someone change out the tissue processor and swap reagents?
- Check the maintenance records for the tissue processor. Talk with the person who changed out the processor.
- Check the reagent bottles to make sure they are all full.
4. What about microtomy?
- Check with the pathologist to see if all the slides are affected – or only a sub-set.
- The issue could have been caused by one technician cutting all their slides too thin.
5. But it is a staining issue – so it must be the stain itself (right?)
- Check the maintenance records for the automated H&E stainer. Talk with the person who changed out the stainer.
- Did any of the stain buckets get swapped?
- Are there new lots of hematoxylin and/or eosin?
- Did a new shipment of alcohol come in?
- What about the clarifier and bluing reagent?
That is a lot of information to assess. Meanwhile, because slides have already gone out to the pathologist, the affected slides must be collected, have the coverslips soaked off and be re-stained…after you have figured out what caused the trouble in the first place. You head to the pathologist’s office, but before you can get through the lab door, the special stains technician needs you to look at a slide.
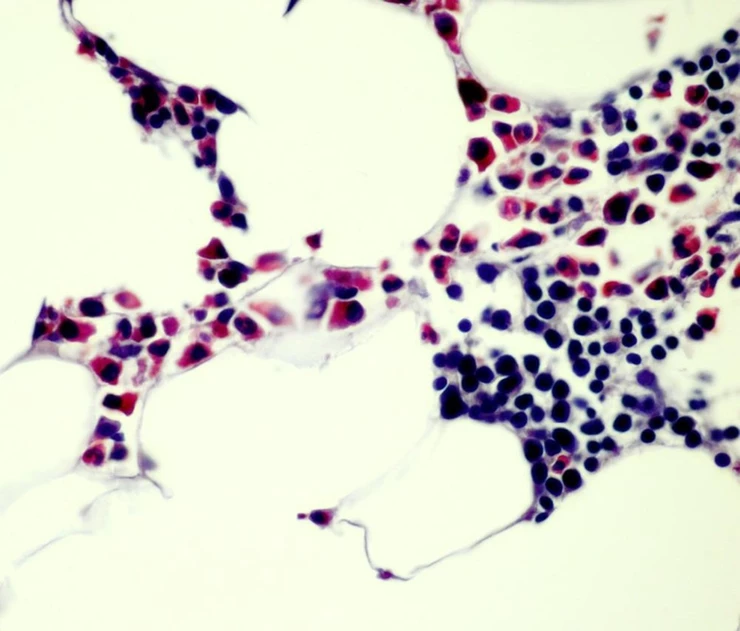
Technologist: “Here is the CAE (chloroacetate esterase) positive control slide that we stained yesterday. The technologist and pathologist checked off on it as staining positively.”
You: “Yes, I agree. The red reaction product is clearly localized within the proper cells.”
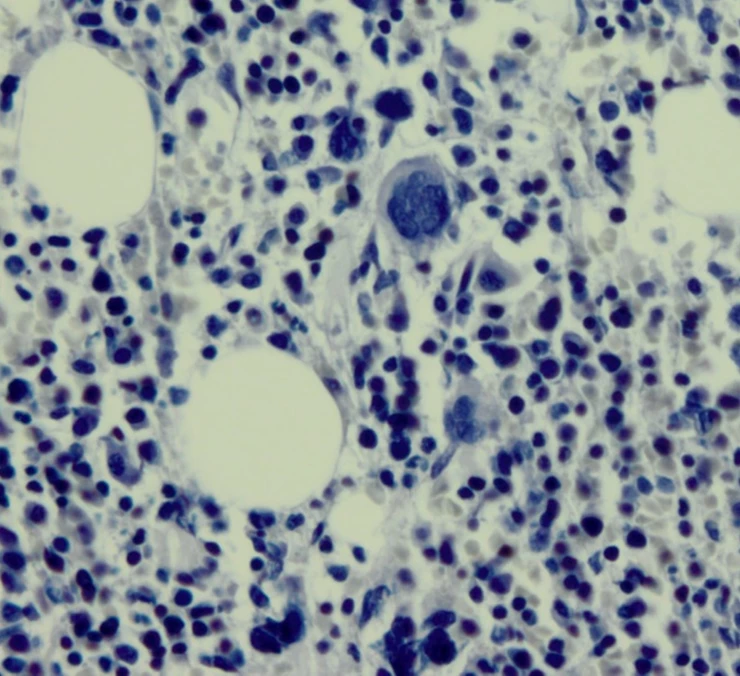
Technologist: “Here is the CAE positive control slide that we stained today. I was checking it before I sent it to the office. I really don’t see the red reaction product.”
You: “Neither do I.”
Technologist: “What do you think happened?”
You: “At this point, I really don’t know.”
You turn around and take the failed CAE slide back to your office. You print out the CAE procedure so that you can start to troubleshoot that as well. You arrived to work today at 6:30 AM; it is now 7:30 AM. You decide that you should call home right now and let them know you will be home late…Again.
But first, you go onto your computer and sign up for the NSH 2021 Convention…
So that you can take Clif Chapman’s presentation: “Troubleshooting Histology: Double Barrel Style -H&E and Special Stains”, along with that molecular technology presentation.
Register between June 8th and Midnight ET on June 14th and take $25 off your registration using discount code BLOG. Register now!


