Before we discuss immunofluorescence, we need to know what fluorescence is. There are certain substances, composed of molecules that will emit light when irradiated by a short wavelength, such as X-rays or ultraviolet (UV) light. The emitted light is of a longer wavelength which has a lower energy. Two substances that are used in microscopy that fluoresce are rhodamine and fluorescein. When rhodamine and fluorescein are irradiated by UV light, they will emit red and green light, respectively.
Fluorescein was first synthesized by Adolf von Bayer in 1871. Later in 1914 Stanislav von Provazek used fluorescent dyes in conjunction with a fluorescence microscope to enhance autofluorescence of cells and tissues. Albert Coons and his colleagues were the first to report using fluorescein to label antibodies to localize the corresponding antigens in tissue sections viewed under a fluorescence microscope in 1942. In 1961 Coons mentioned in a seminar that “the hour of the fluorescent antibody” had started. This is considered to be the beginning of the era of immunofluorescence staining in biology.
Almost eighty years later, the immunofluorescence technique is still used for basic research and patient diagnosis. This author used direct immunofluorescence in research experiments to localize histaminase activity at the cellular level in the 1970’s. Similarly, this author has used direct immunofluorescence for many years in dermatopathology applications for diagnosis of immune diseases.
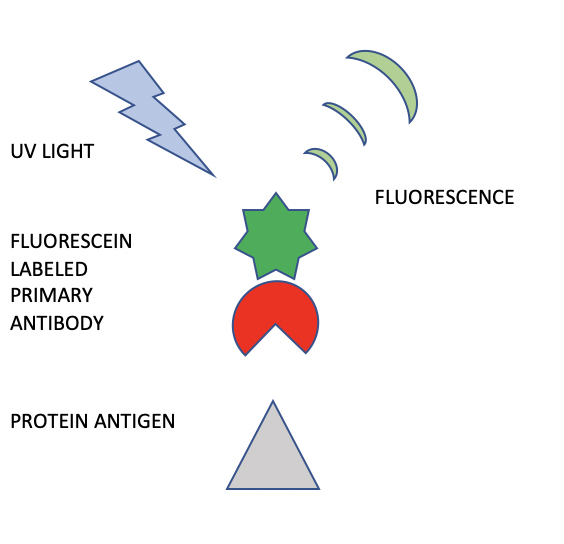
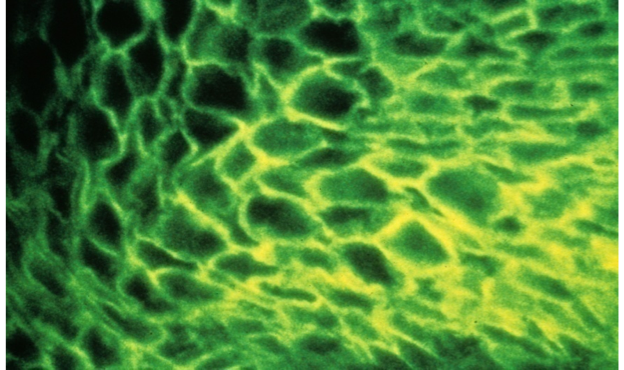
A schematic diagram depicts how direct immunofluorescence (DIF) is performed (Figure 1). A specific antibody against a specific protein is obtained by immunizing a mammal such as a rabbit or mouse. Once the animal is producing circulating antibodies, these can be obtained by taking blood specimens and isolating the desired antibodies. This antibody preparation now undergoes a biochemical process in which molecules of fluorescein are attached to the antibody. This “fluorescein labelled primary antibody” is applied to a tissue section on a microscope slide and incubated for a time period to allow the antibody to attach and bind to the specific antigen under study. After rinsing the slide and coverslipping with aqueous mounting medium, the slide is viewed with a darkfield fluorescence microscope. The slide is irradiated with UV light. The cellular locations of specific antibody staining can be seen as bright “apple-green” fluorescence as shown in Figure 2. Pathologists interpret the various patterns and specificities of stains to determine the patient diagnosis.
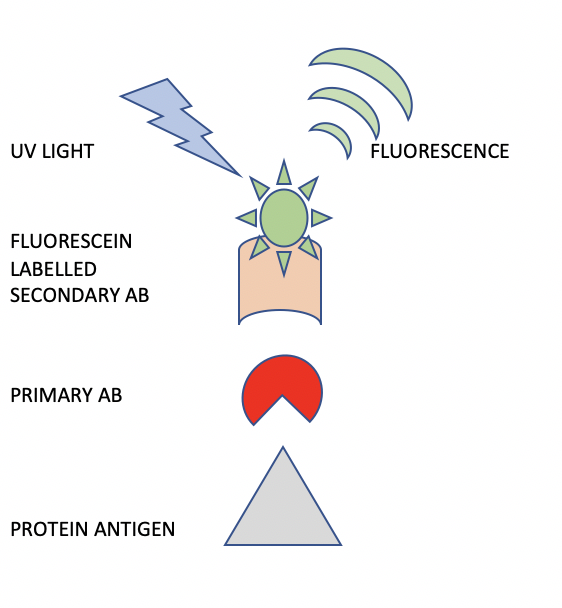
A schematic representation of the indirect immunofluorescence (IIF) procedure is shown in Figure 3. The difference between IIF and DIF is that in IIF, a primary antibody is used alone, followed by a secondary antibody which is labeled with fluorescein. This two-step staining procedure provides great flexibility in the method. For example, an entire panel of rabbit primary antibodies can be localized using only one secondary antibody directed against the rabbit species of primary antibody.
For diagnosis of autoimmune diseases, the primary antibody can be obtained from the patient’s blood serum. When this IgG fraction binds to the appropriate antigen, it can be visualized using a fluorescein labeled anti-human IgG secondary antibody. The resulting staining intensity and pattern can be interpreted by the pathologist to determine the diagnosis.
In an evolutionary development of the IIF stain, cytochemical staining of peroxidase activity, as reported by Graham et al. (1965), was subsequently developed as an immunohistochemical approach by Sternberger et al. in 1970. Sternberger was able to use peroxidase enzyme labelled antibodies to localize antigens and visualize them in the light microscope. This resolution has moved from the tissue to the intracellular level, and the technique is now used for subcellular localization in even the smallest cells. However, the basic idea remains unchanged, almost seven decades after its first description. We shall look at this method in detail in the next part of the series.
References:
1.Chapman CM. The Histology Handbook. Amazon CreateSpace Independent Publishing Platform; 2017
2. Chapman CM, Dimenstein IB. Dermatopathology Laboratory Techniques. Amazon CreateSpace Independent Publishing Platform; 2016
3. Lin CW, Chapman CM, DeLellis RA, Kirley SD. Immunofluorescent staining of histaminase (diamine oxidase) in human placenta. J Histochem Cytochem 26:1021, 1978
4. https://www.nature.com/milestones/milelight/full/milelight02.html
5. Coons, A. H., Creech, H. J., Jones, R. N. & Berliner, E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J. Immunol. 45, 159–170 (1942) | ChemPort |
6. Sternberger, L. A., Hardy, P. H. Jr, Cuculis, J. J. & Meyer, H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-anti-horseradish peroxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 18, 315–333 (1970)
7. Graham, R. C. Jr, Lundholm, U. & Karnovsky, M. J. Cytochemical demonstration of peroxidase activity with 3-amino-9-ethylcarbazole. J. Histochem. Cytochem. 13, 150–152 (1965) | PubMed | ISI | ChemPort


