In the previous segment, immunofluorescence methods were discussed. These methods use antibodies labelled with fluorescein to directly localize cellular proteins in tissue sections, which can then be visualized in a dark field fluorescence microscope. Despite the advantages of this method, science is always in search of newer methods to gain more knowledge. Thus in 1970 Sternberger et al (1970) reported an improvement of Graham’s method (1965) using horse radish peroxidase enzyme (HRP) labelled antibodies to localize antigens and visualize them in the routine light microscope – bringing the method “to light” as one might describe. Now researchers and pathologists could see protein localization within the histology of formalin fixed paraffin embedded tissues viewed using a routine brightfield microscope. This provided vast improvements over the immunofluorescence method.
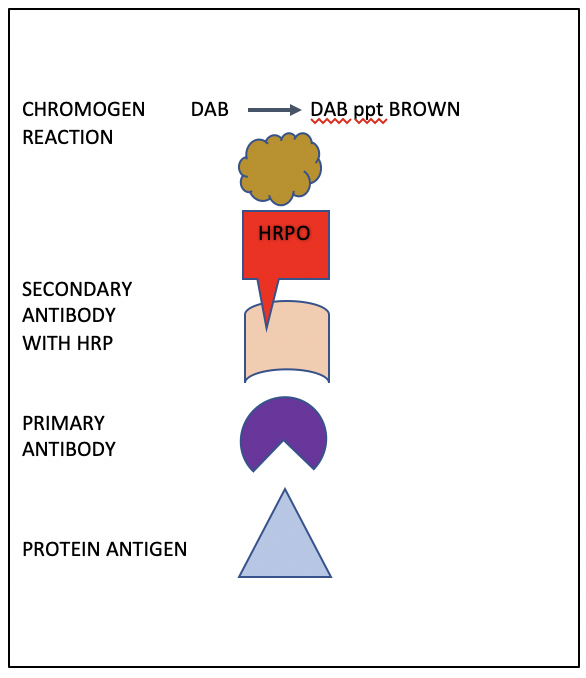
Figure 1 shows a schematic representation of Sternberger’s indirect immunoperoxidase method. Starting at the bottom, a primary antibody (purple) directed against the antigen in question located in the tissue section (blue triangle) is applied to the microscope slide which has been deparaffinized and hydrated. Once the primary antibody incubation is complete, the slide is washed with buffer, and then a secondary antibody is added (tan). The secondary antibody is conjugated to horse radish peroxidase enzyme (red). After washing, the slide is incubated with a diamino-benzidine (DAB) chromogen solution. Wherever the peroxidase is located, the DAB precipitates out. The final result is dark brown staining at the site of the antigen localization.
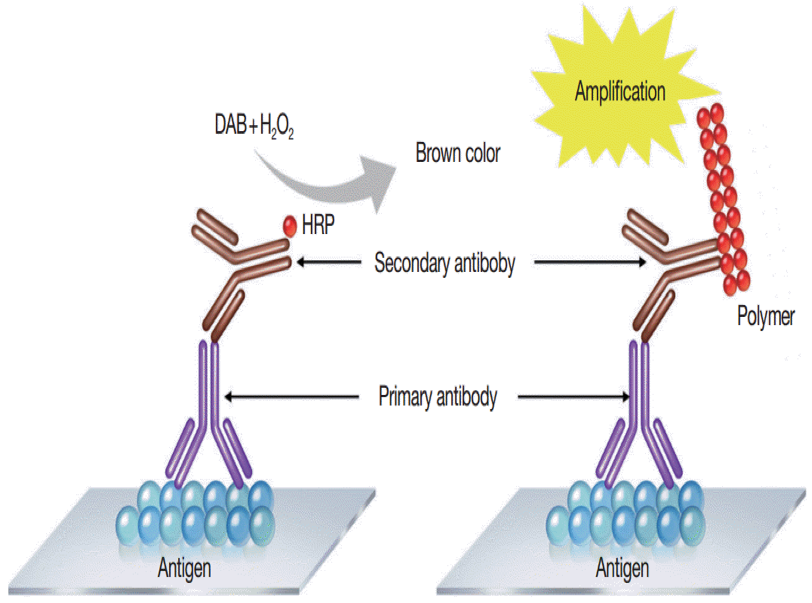
Since the majority of research was done on human tissue, one disadvantage of the Sternberger technique was that the secondary antibody was “anti-human IgG”, and therefore might bind to endogenous human IgG present in the tissue section, thereby causing background staining. To eliminate this possibility, researchers used biotin to label the secondary antibody followed by an avidin-HRP complex. This decreased most non-specific binding. However, in tissues high in biotin (i.e. liver) the possibility was now that the avidin could bind non-specifically to that endogenous biotin and cause additional background staining. This was finally solved by the methods currently used in which polymers are used as the linking reagent and HRP (or other enzyme) carrier. Figure 2 shows this current method as compared to the original Sternberger method.
In summary, direct immunofluorescence (DIF) techniques use a primary antibody that is directly conjugated to the fluorescein label. This causes a one-to-one, signal-to-antigen result and must be viewed in a dark field microscope. The indirect immunoperoxidase of Sternberger, as currently modified using polymers, results in many signals to one antigen, thereby causing the staining to be increased. This technique, combined with the ability to view the results in a routine brightfield microscope, is a great improvement.
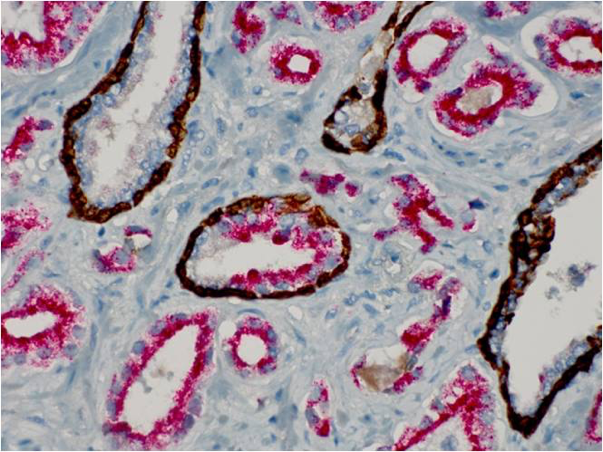
It is important to remember that these techniques must incorporate blocking reagents of some sort, in order to eliminate unwanted non-specific staining in the final slide. Likewise, the use of high purity antibody preparations also decreases the chances of background staining. There is even the ability to label more than one antigen in a single tissue section. Figure 3 shows multiple staining in a prostate needle biopsy exhibited in exquisite fashion. The use of immunohistochemistry in diagnostic pathology has greatly increased the available information from with to make diagnoses and prognoses. We will see in the next unit that the method can be refined even further.