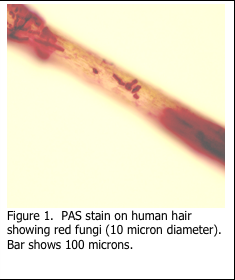
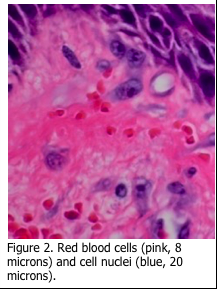
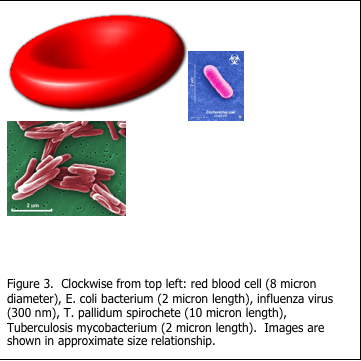
The staining of microorganisms in histology can be challenging. Filamentous fungi and associated conidia are more easily demonstrated as they are visible under light microscopy when stained with periodic acid Schiff’s (PAS) as in Figure 1. The diameter of fungi filaments is 5-10 microns, which is approximately the same as the diameter of a red blood cell, while their length may be hundreds of microns (Figure 2). Microorganisms are extremely small and are at the limit of resolution of the light microscope. Viruses are even smaller (Figure 3).
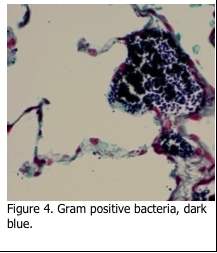
Bacteria exist in three different shapes. Cocci are round, bacilli appear as rods and spirochetes are corkscrew shaped. The first level of determination for bacteria is whether they are Gram positive or Gram negative. The original method of Gram’s technique published in 1884 is still used today. Tissue sections are first stained with a crystal violet solution, which stains all of the tissue section. This is followed by Gram’s iodine which locks the crystal violet stain into the cell wall of the bacteria. Subsequent decolorization with acetone removes the stain from everything – except the Gram “positive” bacteria, which remain a dark blue/purple color (Figure 4).
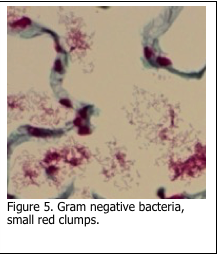
The second phase of the stain involves the application of fuchsin / neutral red solution which stains Gram “negative” bacteria pink/red (Figure 5). This can be the most variable step in the procedure and relies on the correct application and retention of the basic fuchsin within Gram negative bacteria.
There are two additional steps which may result in variable staining as well. The first is an over-decolorization with acetone, which may result in faint staining of Gram positive bacteria. The second is the use and timing of the final counterstain, which may be either picric acid/acetone or tartrazine. While picric acid/acetone results in a more reliable result, the picric acid is a dangerous chemical and requires specific disposal procedures. Tartrazine is safer, but can easily be leached out during the dehydration process prior to coverslipping. The histotechnician performing this stain must rely on experience and precise timing to produce consistent results for this stain.
Actinomycetes are a Gram positive, non acid fast, branching filamentous organism. However, care must be taken when performing the Gram positive stain sequence. It may be necessary to (a) increase the time in crystal violet solution and (b) decrease the time in the acetone decolorization step in order to demonstrate the organism.
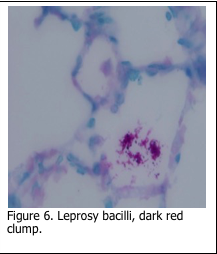
A second determination of bacteria is based on an organisms “acid fastness”. The Kinyoun method for acid fast bacteria, the Fite method for leprosy bacilli and the Ziehl-Neelsen stain for tubercule bacilli are all based on the same stain theory. Tissue sections are first stained with a carbol fuchsin solution, which stains all tissue elements pink/red. Subsequent decolorization in an acid-alcohol solution removes stain from all the tissue elements, except acid fast bacteria. Methylene blue is usually used as a counterstain (Figure 7).
Spirochete bacteria are ubiquitous in nature. They are found everywhere in soil and water. The issue is that some are pathogenic to humans. TheTreponema pallidum spirochete is sexually transmitted and causes syphilis. The Borrelia burgdorferi spirochete is present in certain tick species, and may be transmitted to humans when the tick attaches to the skin to feed. The result is Lyme disease, which if left untreated, can be debilitating to the patient.
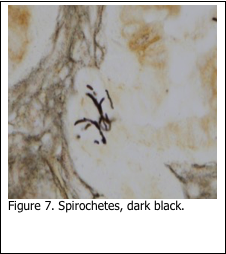
Silver stains were developed in the early 1900’s to stain spirochetes. The Dieterle (1927), Warthin-Starry (1920) and Steiner (1944) are still used in histology today to demonstrate spirochetes. Some of the methods have been modified to make them safer to use (Margeson and Chapman, 1996). The stain theory is the same in all methods: pre-treat the sections to make the spirochetes more readily able to pick up and bind the silver solution. The final result is to stain the spirochetes black, against a gray/ brown background (Figure 7). Those who have performed any of these stains are aware of the many staining procedure pitfalls, which can render the spirochetes either over or under stained. As a result, many laboratories currently use immunohistochemistry to stain spirochetes. While both methods can stain spirochetes, neither is able to demonstrate the exact species to which the stained organism belongs.
Both positive and negative control slides should be used when performing stains for microorganisms. The negative control slide ensures that there is no bacterial contamination present in source water or stain solutions.
References
- Theory and Practice of Histological Techniques. Chapter 10. JD Bancroft, A Stevens ed. Churchill Livingstone, NY. Fourth edition. 1996
- Theory and Practice of Histotechnology. Chapter 9. DC Sheehan, BB Hrapchak. CV Mosby Company, St. Louis. First edition. 1980.
- Margeson LM, Chapman CM: The use of zinc formalin as a sensitizer in silver stains for spirochetes. J Histotech, 19:135-138, 1996.


