In the previous blog we looked at one way to minimize troubleshooting by being proactive and looking ahead to possible situations and procedures that exist in your laboratory that may cause sub-optimal events. This blog will continue with that same mind set.
Some specimens may be received in formalin into the routine lab; however, they may require “non-routine” handling. Skin specimens submitted for “Slow Mohs” processing are such specimens. These are pieces of skin that are carefully marked with colored inks to indicate exact orientation. Laboratory personnel who receive the specimens, and who perform the surgical grossing techniques must recognize these “up front”. Failure to recognize such specimens may result in changing/destroying the orientation, resulting in the pathologist’s inability to render an accurate diagnosis regarding tumor location and surgical margins.
Similarly, a 2 mm punch specimen of skin may be mishandled by laboratory staff, if not identified “up front” at the time of receipt. These specimens should be identified for the embedding and microtomy personnel. The microtomy personnel must pick up the very first sections and observe the unstained slide under the microscope to ensure correct dermal-epidermal orientation (Figure 1A, B). If the specimen has been mis-embedded, there is enough tissue left to melt down and re-embed correctly. Failure to follow this procedure may result in the production of initial and level slides that are mis-embedded. The result may be that the specimen cannot be diagnosed. This would be a possible troubleshooting event that could not be remedied, with dire consequences.
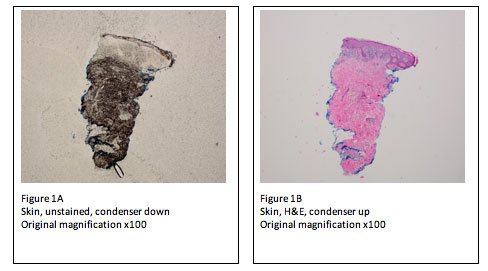
The procedure of “racking down” the condenser to view unstained sections can be used by the microtomist to determine “full-face” sections, presence of knife marks, tearing of sections and overall section quality prior to staining. These issues are best addressed “up front” when they can be remedied, rather than sending suboptimal slides to the pathologist. Microtomists can now more fully control the quality of the sections that are submitted to the pathologist. This procedure also decreases the chances of a suboptimal event related to tissue section quality.
There are some unique specimens received in the histology laboratory that may cause sub-optimal events for the histologist if not handled properly at the time of surgical grossing. Skin specimens may be taken from the scalp to determine if the patient is suffering from alopecia (hair loss). These are almost always punch specimens and are usually 3-4 mm in diameter. To count hair follicles for comparison, these are the only skin specimens that are not cut perpendicular to the dermal-epidermal junction. Rather, they are cut parallel to it, resulting in cross sections of the hair follicles. This method was developed by Dr. Headington and is referred to as the Headington procedure. Some clinicians may submit two punch specimens: one for the Headington procedure, and the other for routine “vertical” sections.
If a skin punch specimen is sent for the Headington procedure, and is mistakenly prepared for a vertical section, there is a remedy. The two pieces of the split punch in the paraffin block can be melted down, positioned so that the specimen is “back together”, then embedded “on end” with the dermis down. This will provide the pathologist with the proper orientation of the Headington procedure.
Another unique specimen which may be sent to your laboratory for evaluation is a punch specimen of skin for diagnosis of painful sensory neuropathy, which can be caused by any number of diseases that affect the peripheral nerves. Usually, a 3 mm punch biopsy is taken for evaluation of density of intraepidermal nerve fibers (IENF) that are reduced in painful sensory neuropathy. The specimen requires 24 hours fixation in 2% paraformaldehyde (Paraformaldehyde/Lysine/Periodate-PLP) fixative. The procedure requires a special protocol of cryosectioning after embedding in sucrose, but at the grossing level of processing, it is necessary to provide adequate fixation. The principles of grossing are the same in Small Fiber Neuropathy (SFN) tests such as Epidermal Nerve Fiber Density (ENFD) and Sweat Gland Nerve Fiber Density (SGNFD). It is extremely important that these specimens are identified “up front” during accessioning to route them into the proper area of the laboratory for specimen preparation.
REFERENCES:
- Chapman, C.M. (2017). The Histology Handbook: In Search of the Perfect Microslide. Amazon CreateSpace Independent Publishing Platform
- Chapman, C.M. and Dimenstein, I.B. (2016). Dermatopathology Laboratory Techniques. Amazon CreateSpace Independent Publishing Platform
- Chapman, C.M. (2018). Troubleshooting in the Histology Laboratory. Submitted to J Histotechnol


